|
|
Research ArticlesDid Warfare Among Ancestral Hunter-Gatherers Affect the Evolution of Human Social Behaviors?
Since Darwin, intergroup hostilities have figured prominently in explanations of the evolution of human social behavior. Yet whether ancestral humans were largely "peaceful" or "warlike" remains controversial. I ask a more precise question: If more cooperative groups were more likely to prevail in conflicts with other groups, was the level of intergroup violence sufficient to influence the evolution of human social behavior? Using a model of the evolutionary impact of between-group competition and a new data set that combines archaeological evidence on causes of death during the Late Pleistocene and early Holocene with ethnographic and historical reports on hunter-gatherer populations, I find that the estimated level of mortality in intergroup conflicts would have had substantial effects, allowing the proliferation of group-beneficial behaviors that were quite costly to the individual altruist.
1 Santa Fe Institute, 1399 Hyde Park Road, Santa Fe, NM 87501, USA.
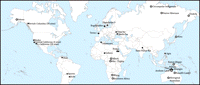
2 University of Siena, Siena 53100, Italy. E-mail: samuel.bowles@gmail.com Intergroup hostilities figure prominently in a number of explanations of the evolution of human social behavior, starting with Darwin ( 1). The underlying mechanism is that (as Darwin put it) groups with "a greater number of courageous, sympathetic and faithful members, who were always ready to warn each other of danger, to aid and defend each other...would spread and be victorious over other tribes" [(1), p. 156]. An implication is that if intergroup conflict is frequent and lethal, then more altruistic group-beneficial behaviors—those entailing greater costs to the individual altruist—will be able to proliferate. Notwithstanding a number of insightful recent studies (2–4), however, lethal intergroup conflict among hunter-gatherers during the Late Pleistocene and early Holocene remains a controversial subject, with little agreement on either its extent or consequences (5, 6). Among the empirical challenges are the lack of written accounts, the difficulty in making inferences from hunter-gatherers in the ethnographic record about conditions before the domestication of plants and animals and the emergence of states, and the fact that most foragers made little use of fortifications and killed each other with the same weapons that they used to hunt other animals, thus leaving few distinctive archaeological traces other than skeletal remains. In light of the available archaeological and ethnographic evidence, could war among ancestral humans have had substantial effects on the evolution of altruistic behavior? To answer the question, I draw upon recent models of human evolution in which competition between groups plays a prominent role (7–14) to quantify the relation between the frequency and intensity of warfare and the selective pressures operating on altruistic behaviors. I use a variant of these models along with a new set of empirical estimates of the extent of war among both prehistoric and historic hunter-gatherers to derive an explicit measure of the importance of warfare in the evolution of human social behavior. This measure is the maximum degree of altruistic behavior—namely c*, the greatest cost borne by individuals in order to benefit fellow group members—that could have proliferated given the empirically likely extent of warfare during the Late Pleistocene and early Holocene. The absence of archaeological evidence of persistent economic and political differentiation between families before about 24,000 years ago (15) indicates that the most informative data for understanding Late Pleistocene humans pertain to hunting and gathering populations without formal political structures (chiefs, "big men," or states). I exclude populations making substantial use of domesticated plants and animals, namely, pastoral, horticultural, agricultural, and equestrian hunting populations. Because hunter-gatherer populations occupying resource-rich areas in the Late Pleistocene and early Holocene were probably sedentary (at least seasonally), I have included wars involving settled as well as purely mobile populations. By "wars" I mean events in which coalitions of members of a group seek to inflict bodily harm on one or more members of another group. The term is not ideal for the ambushes, revenge murders, and other kinds of hostilities likely to have occurred between ancestral groups of humans. Most hostile intergroup contact among hunter-gatherers was probably ongoing or intermittent, with occasional casualties, more akin to boundary conflicts among chimpanzees (16) than to the pitched battles of modern warfare. Using these definitions and selection criteria, I studied all available archaeological and ethnographic sources that present (or are cited as presenting) relevant data. Of these 34 sources, 14 were found to present data that were unrepresentative (for example, when warfare was primarily with modern agricultural populations), unreliable, or inadequate. In three cases, re-estimation of the critical information was possible. Skeletal evidence from sites with fewer than 10 individuals was also excluded. Possible biases in this data set are discussed below. The 8 ethnographic and 15 archaeological sources included yield similar results (Table 1), consistent with the view that prehistoric warfare was frequent and lethal, but somewhat less so than estimates based on data in the standard source for these estimates (6). The populations studied appear in Fig. 1. [Details and additional caveats concerning these and the data to follow appear in (17).]
Intergroup conflict and the evolution of social behaviors. Although both genetic and cultural transmission are probably involved in the evolution of altruistic behaviors, I model only the former, not because it is more important but because it presents greater challenges. (I comment on extensions to cultural transmission below.) The primary behaviors thought to have been spread by war are what Darwin termed the "social and moral qualities" and other forms of altruism. This paradoxical role of war arises because, in the absence of within-group positive assortment, altruism will suffer adverse within-group selection. But it might be sustained by the between-group selection pressures that warfare introduces if altruists willingly fight on behalf of others in their group so that otherwise comparable groups with many altruists tend to prevail in intergroup contests. In game theoretic terms, defense or predation is a public good (participating is an n-person prisoner’s dilemma) in which those who participate confer benefits on their fellow group members at a cost to themselves. While I treat the case of the altruist as warrior as paradigmatic, willingness to take mortal risks as a fighter is not the only form of altruism that contributes to prevailing in intergroup contests; more altruistic and hence more cooperative groups may be more productive and sustain healthier, stronger, or more numerous members, for example, or make more effective use of information. The two key determinants of the effect of warfare on the evolution of social behaviors are the extent of genetic differences between the winners and losers of conflicts and the effect of the number of altruists in a group on group members’ average fitness. Warfare affects the second by making the presence of altruists in a group critical to the members’ survival (and hence their fitness). There are two ways in which the outcome of a conflict may affect the average fitness of its members. The first is that members of losing groups are more likely to perish, and those who die may either produce no offspring or leave children who suffer high mortality due to inadequate parental care. The second is that, as with chimpanzees (18), weaker groups cede territory, thereby redistributing fitness-relevant resources between the groups. I consider a large population made up of subpopulations that periodically engage in hostile contests and study an altruistic behavior that is costly to the individual and has no beneficial effects for group members other than increasing the group’s probability of prevailing in intergroup contests. Groups are sufficiently large that the increased probability of group success in conflict that is associated with an additional altruistic member does not compensate the individual for the cost of the behavior in question. Thus, adopting the altruistic behavior decreases the expected fitness of an individual (by comparison to an individual eschewing the behavior) while increasing the expected fitness of other group members ( 19). For simplicity, I represent the altruistic behavior in question as the expression of a single allele and let individuals reproduce asexually; the model is readily extended to any form of vertical transmission, including cultural.
Modeling warfare and conditions under which altruism may evolve. Following (12), suppose that in every generation with probability
Let pij = 1 if individual i in group j is an altruist, with p ij = 0 otherwise. Let pj be the fraction of group j’s membership that are altruists, p and p' be the altruist-fraction of the metapopulation in a given and subsequent generation, respectively, and
The terms var(pj) and E{var(pij)} are, respectively, the between-group and within-group genetic variance. (E{} indicates a size-weighted average over groups.) The coefficient βG is the effect of variation in pj on the average fitness of members of group j (wj), which (see above) is
The coefficient βi is the effect of variation in pij (namely, switching from a nonaltruist to an altruist) on the fitness of an individual in group j (wij):
where –c is the direct fitness effect of adopting the altruistic behavior and the second term is the indirect positive effect on the individual’s fitness that results from the group’s greater probability of prevailing in a contest. This indirect effect is (dw j/dpj)(dpj/dpij) and is derived using Eq. 2 and dpj/dpij = 1/n, where n is group size (number of individuals in a single reproducing generation in the absence of reproductive skew, fluctuations in group size, and nonrandom migration).
Wright’s inbreeding coefficient FST is the ratio of between-group to total genetic variance [
which says that the extent of genetic differentiation among groups must be greater than the ratio of the costs of the altruistic behavior (the within-group selection pressure) to the benefits (the between-group selection pressures). Equation 4 is a multilevel selection analog to Hamilton’s rule for the proliferation of altruism by kin selection. With these results, the condition for an altruistic allele to proliferate (Eq. 4) can be written as
Rearranging Eq. 5, I define the critical value c* as the maximum cost of the altruistic behavior consistent with its proliferating in the population:
To estimate c*, one needs to know how frequent and how lethal intergroup conflicts were. The richest source is the skeletal evidence studied by archaeologists. Archaeological evidence. As with all archaeological data, it is difficult to establish if the sites that have been studied are representative of Late Pleistocene and early Holocene conditions. As these sites involve burials, they are almost certainly not representative in one respect: Simple disposal of the dead (rather than burial) appears to be typical of the archetypal so-called immediate return foraging group (21). There may be more than accidental bias in the burials studied for signs of violence, given that evidence of violent deaths may be deemed more interesting or worthy of publication than the absence of such evidence. Evidence on given individuals is also incomplete, leading to the opposite bias. Most skeletal remains are never found, and those that are range from intact to fragmentary or poorly preserved, often consisting of just a few of the 100 or so bones in an adult human (excluding the small bones of the hands and feet). The remains of 2185 prehistoric people from Californian sites are accessible to researchers in a museum collection that totals only 12,044 bones (excluding hands and feet); more than 90% of the individuals’ bones are absent (22). Moreover, although some osteological evidence is indicative of ongoing intergroup violence (simultaneous burials, severed limbs, and other evidence of trophy taking, for example), one cannot always distinguish between deaths due to intergroup violence and that occurring within groups. Other biases may lead to underestimates. Many deaths in warfare do not leave projectile points embedded in bone or other traces of violent death: "an analysis that included only projectile points embedded in bone would miss over half of the projectiles...and 75 percent of what was in all probability the actual number of projectile wounds" (23). Studies of arrow wounds treated by U.S. Army surgeons during the Indian Wars found that fewer than a third of the arrows struck bone (24) and that 61% of fatal arrow wounds were to the abdomen (25). Finally, fatalities during combat may fall far short of the total effect of warfare when account is taken of the mortality and reduced reproductive success occasioned by the displacement of the surviving losers. Table 2 gives the resulting estimates.
Ethnographic evidence. Most ethnographic studies of premodern war have concerned populations whose unusually bellicose relations among groups may not reflect conditions of Late Pleistocene hunter-gatherers: horticultural peoples in the highlands of Papua New Guinea and parts of lowland South America, or equestrian hunters or sedentary horticulturalists in North America. Among nonequestrian foragers, detailed accounts provide examples of intergroup conflict of exceptional brutality among Aboriginal Australians, Eskimos, and other groups (3, 26, 27), but most do not allow quantitative estimates of the resulting mortality. In other groups, war is entirely absent from the ethnographic record, but in some of these cases, like the !Kung and other Southern African groups, this absence may be the result of recent state interventions (28, 29). For eight populations, ethnographic studies allow estimates of the deaths due to warfare as a fraction of total mortality (summarized in Table 2). As in the case of archaeological studies, selection bias may lead to an exaggeration of the extent of warfare mortality. Moreover, some populations are not entirely representative of foragers during the Late Pleistocene due to the impact of non–hunter-gatherer influences.
Calibrating the model with hunter-gatherer data. To estimate c*—the maximal direct individual cost of a group-beneficial behavior that could have proliferated—I translate our estimated per-generation mortality rates into an equivalent frequency of decisive conflicts in which the entire territory of a group is taken by the winners, and the losing population is eliminated. This allows me to treat the territorial losses and mortality in a consistent way, and to maintain a constant group size, greatly simplifying the analysis. The data on mortality provide an estimate of
We need two additional pieces of information: the effect of additional altruists on the probability of group survival (
Available estimates of FST for hunter-gatherer populations measure the extent of genetic differentiation both among subpopulations in a given ethno-linguistic group (e.g., among the !Kung in Botswana) and among subpopulations in more than one ethno-linguistic group (e.g., among 18 ethnic groups in Southern Africa). Because prehistoric warfare probably was most common on the boundaries of an expanding ethno-linguistic unit, the latter measure may be the more appropriate one for this study. Excluding those populations that currently live at such a distance from another that it is unlikely that they interacted in the distant past and those that are not at least somewhat reproductively isolated from non–hunter-gatherer populations, there is a total of 18 estimates among hunter-gatherer groups (17). The mean FST of the 18 estimates is 0.074, whereas that for the 15 estimates between ethno-linguistic groups is 0.078. The median for both sets is 0.075. Differences in the genetic material and statistical methods on which these estimates are based make direct comparisons difficult [the Pygmy and Arnhem Land estimates are based on microsatellite data and as a result are likely to be underestimates (30, 31)]. In the illustrative calculations below, I use the median and range of the estimates for between–ethno-linguistic group differentiation.
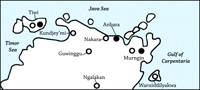
Results. I can now answer the question with which I began: What is the maximum cost of altruism (c*) such that the group benefits would offset the within-group selection pressures against the altruists? To decide whether the resulting values of c* are "large" or "small," note that c* = 0.03, for example, is a quite substantial cost, one that in the absence of intergroup competition would lead the fraction of altruists in a group to fall from 0.9 to 0.1 in just 150 generations. An illustration more directly related to the question of warfare is the following. Suppose that in every generation, a group is engaged in a war with probability  = 2 = 2 and that an altruistic "warrior" will die with certainty in a lost war and with probability 0.20 in a war in which the group prevails, while nonaltruistic members also die with certainty in lost wars but do not die in won wars. (These mortality assumptions are extremely unfavorable for the altruists.) Assuming the altruists have no reproductive advantages during peacetime, then c = 0.2 and that an altruistic "warrior" will die with certainty in a lost war and with probability 0.20 in a war in which the group prevails, while nonaltruistic members also die with certainty in lost wars but do not die in won wars. (These mortality assumptions are extremely unfavorable for the altruists.) Assuming the altruists have no reproductive advantages during peacetime, then c = 0.2 , or (using the mean estimate of , or (using the mean estimate of  from Table 1) c = 0.028. from Table 1) c = 0.028. To study the evolutionary consequences of warfare under Pleistocene conditions using recent data, one would ideally use estimates of both genetic differentiation and wartime mortality from hunter-gatherer populations living in close proximity with one another but having little contact with farmers or herders. Such groups exist in Arnhem Land, Australia, the continent thought by many to be the best laboratory of likely Late Pleistocene and early Holocene conditions among foragers (32). Depictions of warriors and battles in the rock art of Arnhem Land populations date from as early as 10,000 years ago (33). The availability of archaeological, ethnographic, and genetic data for this region makes it a remarkable laboratory for this investigation Fig. 3.
Table 3 presents data on the extent of wartime mortality in three nearby groups of foragers—the Anbara, Murngin, and Tiwi—along with estimates of genetic differentiation among seven Aboriginal groups (including the Tiwi and Murngin) in that relatively small area (34). The estimates of c* for these populations (assuming
To explore the importance of variations in  A, Fig. 2B uses the extreme FST values for differentiation between ethno-linguistic groups (Native Siberian and Pygmy) and the median of these values (South African) to show the combinations values of A, Fig. 2B uses the extreme FST values for differentiation between ethno-linguistic groups (Native Siberian and Pygmy) and the median of these values (South African) to show the combinations values of  A and A and  such that the between-group selection would offset a c* = 0.03. Figure 2B indicates that for plausible values of the effect of altruistic behaviors on a group’s chances of prevailing in contests ( such that the between-group selection would offset a c* = 0.03. Figure 2B indicates that for plausible values of the effect of altruistic behaviors on a group’s chances of prevailing in contests ( A), the levels of warfare mortality observed in many populations would offset substantial costs of altruism. A), the levels of warfare mortality observed in many populations would offset substantial costs of altruism. Discussion. The mortality data summarized in Table 1 are consistent with what is known about the Late Pleistocene from more indirect data. Frequent lethal intergroup encounters may reconcile two otherwise anomalous facts about hunter-gatherer demographics. Human population grew extraordinarily slowly or not at all for the 100,000 years prior to 20,000 years before the present (35, 36), yet under peaceful conditions foraging populations are capable of growth rates exceeding 2% per annum (37, 38). Further, the extraordinary volatility of climate during the Late Pleistocene (39) must have resulted in natural disasters and periodic resource scarcities, known strong predictors of intergroup conflict among hunter-gatherers in the historical record (40), and undoubtedly forced long-distance migrations and occasioned frequent encounters between groups having no established political relations. The mortality data from Southern California (23) and Nubia (41) are consistent with this hypothesis. The evidence that intergroup conflict may have contributed significantly to the proliferation of a genetic predisposition to behave altruistically does not mean that it did, or that the mechanism I have described explains the evolution of human altruism. The model applies with even greater force to behaviors transmitted culturally rather than genetically, in part because between-group differentiation is considerably greater and hence the evolutionary impact of differential group success in contests is stronger. One cannot say with certainty which of these data should be the basis for our conclusions concerning the evolutionary impact of lethal intergroup competition during the Late Pleistocene and early Holocene. Even though periods of climatic volatility would bring even quite distant groups into contact during migrations, the far-flung settlements of the circumpolar regions, desert Southern Africa, and Western Australia would be far less likely to be in contact—either conflictual or beneficial—than groups living in closer proximity such as those in coastal Arnhem Land and lowland New Guinea. Moreover, the more populated coastal and riverine areas contributed disproportionately to the gene pool of subsequent generations. But taking all of the evidence into account, it seems likely that, for many groups and for substantial periods of human prehistory, lethal group conflict may have been frequent enough to support the proliferation of quite costly forms of altruism. This might help explain why altruism often does not extend across group boundaries, and how this kind of "parochial altruism" may have evolved in humans (13) and perhaps even other animals. Because humans are far from unique in the extent of lethal intergroup conflicts (42) and because genetic differentiation among populations of some other "warlike" animals may not be very different from that among humans (43), there remains the as-yet unexplored possibility that a similar evolutionary dynamic may occur in other animals.
Supporting Online Material
www.sciencemag.org/cgi/content/full/324/5932/1293/DC1 Materials and Methods Tables S1 to S5
References and Notes
References and Notes
Received for publication 5 November 2008. Accepted for publication 10 April 2009.
The editors suggest the following Related Resources on Science sites:In Science Magazine
THIS ARTICLE HAS BEEN CITED BY OTHER ARTICLES:
|
Science. ISSN 0036-8075 (print), 1095-9203 (online)

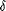 p
p  p' – p. Then, using the Price equation (
p' – p. Then, using the Price equation (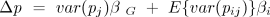
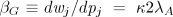
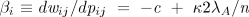
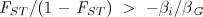
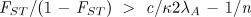
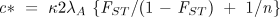
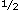 ; so
; so